Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 (am) solubility at 25
(am) solubility at 25 C in the Ca
C in the Ca -Na
-Na -H
-H -Cl
-Cl -OH
-OH -H
-H O system; A Critical review
O system; A Critical reviewRai, D.*; Kitamura, Akira; Altmaier, M.*; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Journal of Solution Chemistry, 47(5), p.855 - 891, 2018/05
Times Cited Count:8 Percentile:8.45(Chemistry, Physical)We have critically reviewed experimental data for Zr hydrolysis constant values for formation of several mononuclear and polynuclear species and a solubility product value for ZrO (am). We have determined new/revised values for the formation constants of Zr(OH)
(am). We have determined new/revised values for the formation constants of Zr(OH)
 , Zr(OH)
, Zr(OH) (aq), Zr(OH)
(aq), Zr(OH)
 , Zr(OH)
, Zr(OH)
 and Ca
and Ca Zr(OH)
Zr(OH)
 , and the solubility product for ZrO
, and the solubility product for ZrO (am) after the critical review.
(am) after the critical review.
Takahashi, Yoshio*; Murata, Miho*; Kimura, Takaumi
Journal of Alloys and Compounds, 408-412, p.1246 - 1251, 2006/02
Times Cited Count:24 Percentile:75.32(Chemistry, Physical)no abstracts in English
Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Minato, Kazuo; Numata, Masami
Proceedings of GLOBAL2003 Atoms for Prosperity; Updating Eisenhower's Global Vision for Nuclear Energy (CD-ROM), p.2285 - 2291, 2003/00
Stability of AmN and (Am,Zr)N was studied comparatively from the viewpoints of the hydrolytic and evaporative behavior. AmN powder reacted with moisture to form hydroxide Am(OH) , while the solid solution (Am
, while the solid solution (Am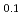 Zr
Zr )N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am
)N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am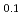 Zr
Zr )N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.
)N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.
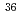 Cl in biological shield concrete using pyrohydrolysis and liquid scintillation counting
Cl in biological shield concrete using pyrohydrolysis and liquid scintillation countingIto, Mitsuo; Watanabe, Kazuo; Hatakeyama, Mutsuo; Tachibana, Mitsuo
Analyst, 127(7), p.964 - 966, 2002/06
Times Cited Count:14 Percentile:40.69(Chemistry, Analytical)A method for the determination of Cl-36 in biological shield concrete of nuclear reactor was developed. Cl in the concrete sample was extracted quantitatively by pyrohydrolysis at 900 ºC and recovered in Na2CO3 solution for subsequent measurement of Cl-36 by liquid scintillation counting. WO3 was used as an accelerator in the pyrohydrolysis. The Cl extraction procedure was optimized by investigating experimental conditions with the use of ion chromatography and its recovery was evaluated by the analysis of the geochemical reference samples. Detection limit of Cl-36 was 0.02 Bq g-1 for sample weight of 2g. Relative standard deviation was 3 – 7 % for the samples containing 0.5 Bq g-1 levels of 36Cl. The newly developed method was applied to determine Cl-36 in biological shield concrete of the Japan Power Demonstration Reactor.
Suzuki, Yasuyuki*; Li, J.; Maekawa, Yasunari; Yoshida, Masaru; Maeyama, Katsuya*; Yonezawa, Noriyuki*
Nihon Kagakkai-Shi, 2002(2), p.255 - 259, 2002/02
The hydrophilic surface of poly(ethylene terephthalate) (PET) film, obtained by partial hydrolysis, was converted to hydrophobic one under dry air, saturated water vapor atmosphere, nitrogen, and vacuum at temperatures ranging from 0 to 80 C. The hydrophilicity of the surface increased significantly faster under the saturated water vapor although it was the most hydrophilic in the examined conditions. From the dependence of the absolute temperature on the rate of hydrophilicity change for each storage condition, a discontinuous point at ca. 50
C. The hydrophilicity of the surface increased significantly faster under the saturated water vapor although it was the most hydrophilic in the examined conditions. From the dependence of the absolute temperature on the rate of hydrophilicity change for each storage condition, a discontinuous point at ca. 50 C was observable only under the water vapor condition. This relation indicates that the appreciable acceleration of the hydrophilicity change on the surface under the hydrophilic condition might be resulted from the increase of the surface mobility due to the water adsorption on the PET surface.
C was observable only under the water vapor condition. This relation indicates that the appreciable acceleration of the hydrophilicity change on the surface under the hydrophilic condition might be resulted from the increase of the surface mobility due to the water adsorption on the PET surface.
JAERI-Tech 98-014, 106 Pages, 1998/05
no abstracts in English
*;
Seni Gakkai-Shi, 45(7), p.318 - 323, 1989/07
no abstracts in English
Nihon Denshi Nyusu, 27(1-2), p.12 - 17, 1987/02
no abstracts in English
; Yamamoto, Katsumune; ; ;
Radioisotopes, 35(12), p.619 - 624, 1986/12
no abstracts in English

; ; ; ;
Radiochimica Acta, 40, p.107 - 111, 1986/00
no abstracts in English
;
Journal of Radioanalytical and Nuclear Chemistry, 84(2), p.269 - 275, 1984/00
Times Cited Count:6 Percentile:57.41(Chemistry, Analytical)no abstracts in English
 -olefin copolymer and its hydrolyzed copolymer
-olefin copolymer and its hydrolyzed copolymer; ; *; Machi, Sueo
J.Appl.Polym.Sci., 24(5), p.1237 - 1245, 1979/00
Times Cited Count:3no abstracts in English
Journal of Radioanalytical Chemistry, 50(1-2), p.133 - 142, 1979/00
no abstracts in English
;
Journal of Nuclear Science and Technology, 15(1), p.50 - 55, 1978/01
Times Cited Count:3no abstracts in English
Journal of Radioanalytical Chemistry, 43(1), p.81 - 91, 1978/01
no abstracts in English
; ; ;
Journal of Nuclear Science and Technology, 4(9), p.482 - 487, 1967/00
Times Cited Count:0no abstracts in English